�
�
�
�
�
�
�
�
�
�
�
�
Inside the
Nickel Metal Hydride
�
�
�
�
�
�
�
�
�
�
�
�
�
�
�
�
John J.C. Kopera
Cobasys
Inside the NiMH
�
Introduction
�
The Nickel Metal Hydride (NiMH) battery has become pervasive in today�s technology climate, powering everything from cellular phones to hybrid electric vehicles.� The NiMH battery started its life as an evolution from the nickel hydrogen battery used in aerospace applications.� Because of their exceptional cycle life and reasonable specific energy, nickel hydrogen batteries were attractive for aerospace applications; however nickel hydrogen batteries have poor volumetric efficiency and require tanks of compressed hydrogen gas and platinum catalysts.� NiMH batteries are the result of configuring a battery using metal hydride hydrogen storage materials as one of the battery electrodes.� NiMH batteries have been in development for well over twenty years, but were mere laboratory curiosities before the development of advanced metal hydride electrodes that were capable of being charged and discharged in a cell environment without failure.� The basic work performed in multi-component metal hydride alloys has paved the way for the current generation of high performance, long life NiMH batteries in use today in nearly every application and those produced by Cobasys.
�
What is a
�
Some definitions are in order here:
�
An electrochemical cell is a chemical reactor containing reactive and electrically conductive materials which react in a controlled manner to produce direct current electricity.
�
In a primary cell or battery the reaction is generally not reversible; i.e. after discharge the cell or battery cannot be recharged by supplying current in the reverse direction, or it may be recharged to only a small fraction of the initial amount of energy available from the first discharge due to the non-reversibility of the chemical reactions inside the cell.� Primary batteries are generally used once and then replaced with new primary cells or batteries.
�
A secondary cell or battery contains chemical substances that allow a reaction in reverse of discharge to occur when charging current is supplied to the cell.� Thus, after a discharge, the cell can be restored to nearly its original amount of energy by application of the charge current in a specified manner.� This discharge/charge activity may occur for several cycles to many thousands of cycles depending on the specific battery technology.
�
The voltage or electrical pressure generated by an electrochemical cell depends on the types of chemicals involved in the reactions, while the energy (in watt hours � Wh) depends on the amounts and nature of the chemicals comprising the cell.
�
The ability of the cell to provide power (in watts � W (voltage x current)) is determined by many factors including the chemical makeup of the cell, cell construction, temperature, etc.
�
A battery is technically a string of electrochemical cells connected in series to achieve a higher voltage.� However, for convenience we shall call a cell and also series and parallel connected cells a battery as is a common convention.� Batteries may be referenced both by the chemistry of the cells as well as the voltage developed.
�
Basic Components of an Electrochemical Cell
�
The anode is the electrode where oxidation takes place and electrons are fed out of the cell into the external circuit.� The cathode is the electrode where reduction takes place and the electrons from the external circuit return to the cell.
�
In a primary cell, the anode is also the negative electrode and the cathode is the positive electrode.� In a secondary cell, when on charge the negative electrode becomes the cathode and the positive electrode becomes the anode.� Because of the reversal of roles in a secondary cell, the electrodes will be referred to as either positive or negative (which never changes) and the direction of current flow (charge or discharge) will be specified.
�
The electrolyte serves as the path for completing the electrical circuit inside of the cell via the transport of ions from one electrode to the other.
�
The reactants making up the electrodes (the active material) may be gaseous, liquid, or solid.� The electrolyte may be a liquid or a solid.
�
Figure 1 shows a schematic of an electrochemical cell on charge and discharge.
�
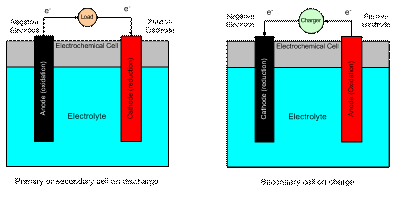
Figure 1.� Electrochemical cell schematic
�
�
�
Basic Components of a Cell
�
Electrodes � must be electronically conducting.� Most of the compounds used for positive active materials (especially oxides) in conventional batteries are not good electrical conductors and therefore must be mixed with or supported by conductive compounds or networks.� These include electrode material additives and the grid or plate structure of the electrode made from such materials as graphite, lead, nickel, copper, etc.
�
Electrolyte � must be ionic conductor.
�
Separator � provides physical isolation between the electrodes to prevent shorting and to separate the electrode reactions from one another.� It also must be able to pass ions through its structure to allow for current flow.
�
Enclosure or packaging � Holds all of the battery electrodes, electrolyte, terminals, vents, etc.
�
�
Ideal electrodes for energy storage in batteries would be made from elements that come from two separate columns of the periodic table that produce good reversible electrochemical reactions of oxidation and reduction.� For example negative electrodes from column 1A such as Lithium (Li), or Sodium (Na) and positive electrodes from column 6A such as Oxygen (O2) and Sulfur (S) respectively (1).
�
Figure 2.� Periodic Table of the Elements
�
Most battery technologies use an electrochemical couple consisting of a column 1A, 2A, 1B, or 2B metal electrode and a metal oxide or a column 6A (or 7A) elemental electrode.� Electrodes such as Air (O2) are created by using a conductive structure with a catalyst for promoting the reaction.
�
The following table shows a comparison of various secondary battery systems along with their theoretical and practical energy densities.� Looking at the following table one notices a vast difference between the theoretical specific energy density and the practical specific energy density of the various battery types.� This disparity is due to the fact that the theoretical energy density only looks at the active materials involved in the electrochemical reaction within the battery and disregards the other components necessary for the battery to function such as electrode structural material, battery enclosure, electrolyte, separator materials, terminals, etc.
�
Table 1. Secondary Battery Technology Comparison (1)
|
|
Negative Electrode |
Positive Electrode |
Electrolyte |
Nominal Voltage (V) |
Theoretical Specific Energy (Wh/kg) |
Practical Specific Energy (Wh/kg) |
Practical Energy Density (Wh/L) |
Major Issues |
|
Lead-Acid |
Pb |
PbO2 |
H2SO4 |
2.0 |
252 |
35 |
70 |
Heavy, Low Cycle Life, Toxic Materials |
|
Nickel Iron |
Fe |
NiOOH |
KOH |
1.2 |
313 |
45 |
60 |
Heavy, High Maintenance |
|
Nickel Cadmium |
Cd |
NiOOH |
KOH |
1.2 |
244 |
50 |
75 |
Toxic materials, maintenance, cost |
|
Nickel Hydrogen |
H2 |
NiOOH |
KOH |
1.2 |
434 |
55 |
60 |
Cost, High Pressure Hydrogen, Bulky |
|
Nickel Metal Hydride |
H (as MH) |
NiOOH |
KOH |
1.2 |
278 � 800 (depends on MH) |
70 |
170 |
Cost |
|
Nickel Zinc |
Zn |
NiOOH |
KOH |
1.6 |
372 |
60 |
120 |
Low cycle life |
|
Silver Zinc |
Zn |
AgO |
KOH |
1.9 |
524 |
100 |
180 |
Very expensive, limited life |
|
Zinc Air |
Zn |
O2 |
KOH |
1.1 |
1320 |
110 |
80 |
Low Power, limited cycle life, bulky |
|
Zinc Bromine |
Zn |
Bromine Complex |
ZnBr2 |
1.6 |
450 |
70 |
60 |
Low Power, hazardous components, bulky |
|
Lithium Ion |
Li |
LixCoO2 |
PC or DMC w/ LiPF6 |
4.0 |
766 |
120 |
200 |
Safety Issues, Calendar Life, Cost |
|
Sodium Sulfur |
Na |
S |
Beta Alumina |
2.0 |
792 |
100 |
>150 |
High
Temperature |
|
Sodium Nickel Chloride |
Na |
NiCl2 |
Beta Alumina |
2.5 |
787 |
90 |
>150 |
High Temperature Operation, Low Power |
�
The NiMH battery has many significant advantages over other rechargeable technologies including cycle life, safety, and non-hazardous materials.� The NiMH battery has continuously evolved over the past 20 years from existence only as a laboratory curiosity to a highly developed product for a variety of applications including consumer products, electric vehicles, hybrid electric vehicles, and stationary power applications.� All commercial NiMH batteries use negative (metal hydride) electrode materials which are patented.� The Cobasys� NiMH batteries utilizing these materials are among the most advanced prismatic NiMH batteries on the market today.
�
The NiMH
�
The NiMH battery is termed an alkaline storage battery due to the use of potassium hydroxide (KOH) as the electrolyte.� Electrically, NiMH batteries are very similar to nickel cadmium batteries.� Rechargeable alkaline storage batteries are a dominant factor in the market for several technically important reasons:
�
�������� High electrolyte conductivity allows for high power applications
�������� The battery system can be sealed, minimizing maintenance and leakage issues
�������� Operation is possible over a wide temperature range
�������� Long life characteristics offset higher initial cost than some other technologies
�������� Higher energy density and lower cost per watt or watt-hour (depending on design)
�
Cobasys� NiMH batteries have been developed to respond to a number of specific market requirements such as recyclability, high power, high energy density, long life, and other important characteristics for consumer and OEM applications.� They are more suitable than almost all other types of secondary batteries in almost all applications where high currents and deep discharges are required.�
�
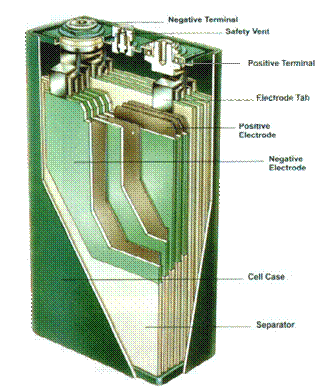
Figure 3.� Cutaway of Prismatic NiMH cell
The electrolyte, which is an aqueous solution of potassium hydroxide, has a very high conductivity and usually does not enter into the cell reaction to any significant extent.� The electrolyte concentration (and therefore a major component of cell resistance) remains fairly constant over the entire range of state of charge or discharge.� These factors lead to a battery with high power performance and long cycle life.
�
The active materials in the NiMH battery are composed of metal compounds or metallic oxides which (in a charged state) are relatively good conductors.� The nickel oxide � hydroxide electrode only exchanges a proton in the charge-discharge reaction and the electron transfer is very rapid contributing to high power capacity.� The small change in size of the electrode between charge and discharge also results in greater mechanical stability and thus longer cycle life.
�
NiMH batteries can be fabricated in virtually any size from tens of milliampere hours to hundreds of ampere hours or more.� Due to the compatibility of steel with the KOH electrolyte, the batteries can be manufactured in steel cans which are very rugged and exhibit good thermal performance.� Large energy storage systems for a variety of applications from transportation to stationary backup power have been successfully applied using NiMH technology.� This versatility is constantly leading to new applications for NiMH batteries where performance and environmental factors are of utmost importance.
�
Positive Electrode
The positive electrode of the NiMH battery is nickel hydroxide.� This is a very well developed electrode material with almost 100 years of history and development since it is the same composition as it is for NiCd batteries.� Nickel based alkaline batteries are attractive since the nickel electrode can be fabricated with very large surface areas which lead to high capacities and high current densities.� The electrolyte does not enter into the electrode reaction so that conductivity stays at a high level throughout the usable capacity of the battery.� In addition the nickel active material is insoluble in the KOH electrolyte which leads to longer life and better abuse tolerance.� Only a proton is involved in the charge/discharge reaction leading to very small density changes and improved mechanical stability of the electrode during cycling.� Also the gravimetric and volumetric energy densities are very good for the nickel electrode.� (2)
�
The simplified nickel electrode reaction in the cell is:
�
![]()
�
The actual reaction is more complicated because of several factors (2):
- The nickel electrode is nonstoichiometric
- The reaction involves proton diffusion in the solid state
- Additives to the electrode affect charge transfer and crystal structure of the electrode
- β-NiOOH is a low conductivity p-type semiconductor when nickel valence is
< 2.25
- Oxidation states if nickel > 3 have been determined to be present (i.e. K(NiO2)3 �→ Ni3.67+)
- Transformations of the NiOOH occur in the KOH solution
�
Negative Electrode
�
The active material for the negative electrode in the NiMH battery is actually hydrogen, the same as it is in a nickel hydrogen battery, except that the hydrogen ions (protons) are stored in the metal hydride structure which also serves as the electrode.� The metal hydride can, depending on its composition, hold between 1% and 7% hydrogen by weight.� As a hydrogen storage material, the metal hydride is very efficient, achieving better volumetric efficiency than liquid hydrogen.� Today�s practical materials for NiMH batteries hold between 1% and 2% hydrogen by weight. (1)
�
Many elemental metal hydride materials exist but were not practical for battery applications due to the high equilibrium pressure exhibited by these materials at room temperature.� This changed when intermetallic compounds were developed that combined strong and weak hydride forming materials.� Tailoring the metal hydrides for the desired equilibrium pressure and other chemical properties is achieved by adjusting the ratio between these two types of material components.
�
Intermetallic compounds are alloys of two or more metallic elements with narrow bands of integer stoichiometries.� The compounds are divided into groups classified by AxBy based on their composition and crystal structure (4).� The A and B components can each consist of a number of different elements in varying ranges of stoichiometry.� The variation of the components of the metal hydride allows the design of materials with the desired characteristics for use in battery applications such as low equilibrium pressure, resistance to corrosion, mechanical stability, reversibility, hydrogen storage ability, etc.� Table 2 shows some of the most common metal hydrides used for battery applications.
�
Table 2.� Metal Hydride Classes and Materials (4)
|
AxBy Class (Basis) |
Components |
Storage Capability (mA/g) |
Comments |
|
AB5 (LaNi5) |
A: Mischmetal, La, Ce, Ti B: Ni, Co, Mn, Al |
300 |
Most commonly used alloy group for NiMH battery applications |
|
AB2 (TiNi2) |
A: V, Ti B: Zr, Ni (+Cr, Co, Fe, Mn) |
400 |
Basis of �multi-component alloys� used in some NiMH battery systems |
|
AB (ZrNi) |
A: Zr, Ti B: Ni, Fe, Cr, V |
� |
Used in early development of hydrogen storage |
|
A2B (Ti2Ni) |
A: Mg, Ti B: Ni |
� |
Note:� Mischmetal is a naturally occurring mixture of �rare earth� elements and other metals.
The Cobasys NiMH batteries use either an AB2 or an AB5 metal hydride alloy for the negative electrode.� The reactions for the negative electrode can be written as:
�
![]()
�
Where, M represents the metal hydride material.
�
The NiMH
�
The complete cell is represented schematically in Figure 4 below.� The combined whole cell reaction can be written as:
�
![]()
�
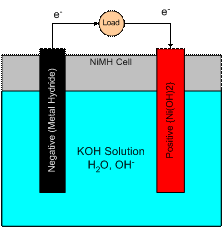
Figure 4.� The NiMH cell
�
Charge and Discharge Characteristics
�
Figure 5 shows a typical charge / discharge curve for the NiMH battery.
�
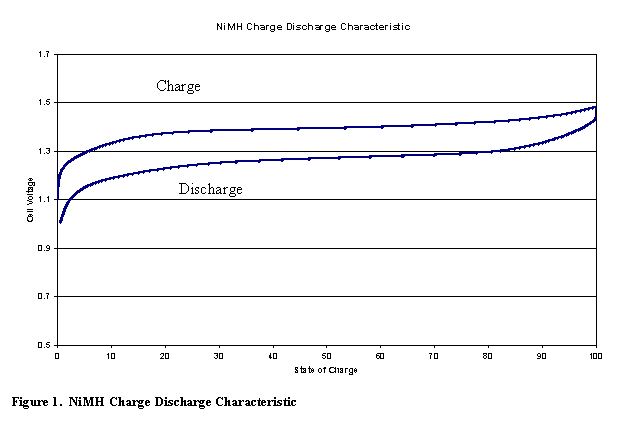
The NiMH battery also has over-charge and over-discharge reactions that allow the battery to handle abuse without adverse effects.� This is called the oxygen cycle for overcharge.� On over-discharge the battery has the hydrogen cycle (1).
�
The oxygen cycle functions as follows on overcharge:
�
For the positive
electrode:� ![]()
For the negative
electrode:� ![]()
Net is no reaction, only heat generation equal to the energy input and an increase in cell pressure.
�
The hydrogen cycle functions as follows on over-discharge:
�
For the positive
electrode:� ![]()
For the negative
electrode:� ![]()
Net result is no reaction but heat and pressure are generated in the cell.
�
In an extreme case of overcharge the cell will become pressurized enough to cause the safety vent to open and release the excess pressure, thus avoiding the danger of cell rupture.
�
The NiMH battery is capable of supplying a large quantity of power to the load.� Specific powers of 200 W/kg to greater than 1000 W/kg are available from Cobasys NiMH products depending on the design and application requirements.� NiMH batteries exhibit a very linear resistance characteristic for discharge and charge at any given SOC.� This makes modeling the battery behavior easy for applications in electric vehicles and other high energy and high power applications.
�
The Cobasys NiMH batteries have demonstrated cycle life of greater than one thousand 80% Depth of Discharge (DOD) charge discharge cycles.� In applications such as hybrid electric vehicles which utilize very shallow charge-discharge cycles 200,000 to 300,000 cycles have been achieved.
�
Self Discharge
�
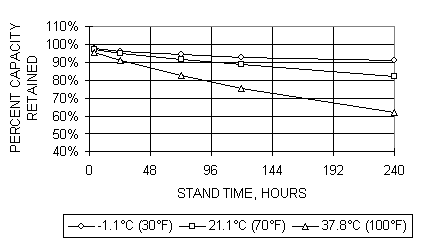
The NiMH batteries, like all batteries, have a certain level of self discharge that occurs when the battery is at rest.� The self discharge characteristic typical of an available electric vehicle type battery module from Cobasys is shown in figure 6.� The factors contributing to self discharge include the energy used by the oxygen cycle at high states of charge.� The contribution to self discharge from the oxygen cycle is negligible below about 70% state of charge.� Longer term contributions to self discharge are caused by chemical ion shuttles which continuously discharge the cell over longer periods of time.� The rate of the self discharge is highly dependent on the temperature of the cell.� Higher temperatures yield higher self discharge rates.
�
Figure 6.� Capacity retention at several temperatures for NiMH EV battery
�
NiMH
�
The NiMH battery has a
wealth of applications from portable consumer products such as digital cameras,
cell phones, etc. to electric and hybrid vehicle applications and industrial
standby applications including energy storage for Telecom,
�

�
Figure 7.�
�
Cobasys� NiMH batteries have been successfully field tested in many electric and hybrid vehicle applications providing millions of miles of road experience to draw upon.� Our batteries enabled the GM EV1 to have a real world range of 250 km and the Chevrolet S10 achieved a range of 110 to 130 km with full payload.� Many other heavy duty vehicle applications in pure electric busses and hybrid electric vehicles also are demonstrating the powerful and long lasting performance of the NiMH battery systems.
�
Standby power systems are also an ideal area of application for the larger NiMH batteries.� They have a variety of advantages over the traditional lead acid batteries that have been traditionally employed in these applications.� The advantages include:
�
��������� Small Footprint
��������� Long Life Cycle Characteristics
��������� Low Maintenance
��������� High Power
��������� Light Weight
��������� Safe
��������� Good Thermal Performance
��������� Configurable Design
�
�
�
�
�
�
�
Figure 8.� Examples of Cobasys Products and Applications
�
The NiMH battery is a
versatile solution for many diverse applications due to its long life,
environmentally friendly materials, high power and energy, and safe
application.� Incredible progress has
been made since the early days of development of NiMH technology to today where
Cobasys is producing the world�s most advanced prismatic NiMH batteries
available proven in a host of demanding applications.
References
�
- D. A. Corrigan, �Introduction
to NiMH
Battery Technology,�June 21, 2002 .
�
�
- �Modern
Battery Technology,� Center for Professional Advancement, 1995, Dr. Forrest A. Trumbore ed.
�
�
- M. A. Fetcenko,
S. Venkatesan , S. R. Ovshinsky, �Selection of Metal Hydride Alloys For Electrochemical Applications,� Electrochemical Society Proceedings Volume 92-5, pp. 141-167.
�
�
- D. Berndt, Maintenance-Free Batteries, 2nd edition, 1997.